What is Carbon Capture and Storage (CCS)?
Following the accident at the Fukushima nuclear power plant, the Japanese government decided to revert back to fossil fuel and combustion based power generation.
Unfortunately the burning of fossil fuels, such as oil and coal, produces large amounts carbon dioxide (CO2) that we know has a significant impact on climate change. As a result of this threat thedevelopment of an effective method to capture and recover CO2 from fixed sources, eg. power plants, has become a pressing issue.
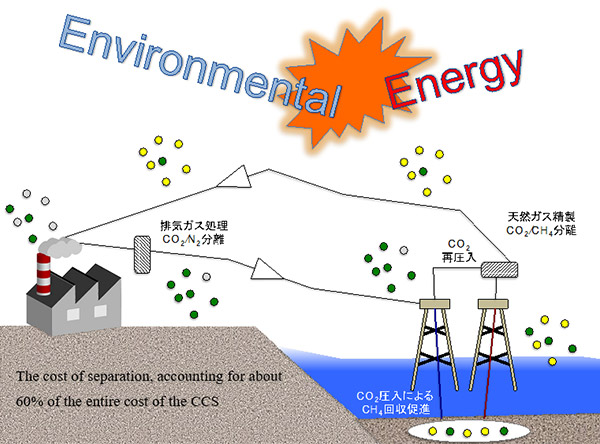
Carbon dioxide Capture and Storage (CCS) is a process to capture, transport, and store CO2 from power plants and factories to appropriate storage sites.
Some attractive forms of CCS are the enhanced oil recovery method (EOR) and CO2-CH4 hydrate substitution method. These techniques can solve two problems at once, the CO2 storage problem and energy production, as it involves using CO2 to displace underground methane (CH4) which can be used as a fuel source.
One of the serious challenges that this type of CCS is facing, is that CO2 separation requires pressurized conditions. The cost of separation, accounting for about 60% of the entire cost of the CCS. An effective way to do CCS can help greatly reduce the cost of gas separation techniques. Among them, as a low-cost method to replace the current separation method, separation by membrane materials has been attracting attention.
In addition to the benefits of separating CO2 gas, the purification of liquefied natural gas requires CO2. This purification is required before the liquefied natural gas can be transported.
Potential of membranes
At the moment, CO2 separation techniques tend to rely on either chemical absorption using chemicals like ethanol amine, or physically based absorption using materials called zeolites.
However, these methods have problems in scaling up for industry and practical use. A big problem is that there is a large energy cost to removing the absorbed CO2 from the absorption material.
On the other hand, when separating gas separation using a membrane method, equipment scale is smaller, the aim for the future is to go small-scale in the natural gas field and industry fields, as a technique that can be applied to power plants, this has attracted a lot of attention.
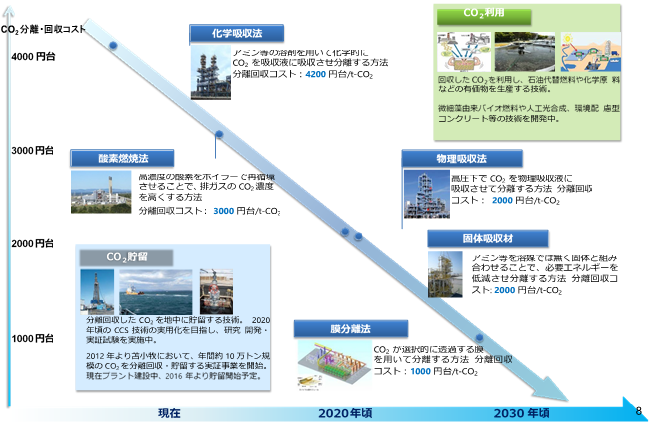
出典:CO2回収、利用に関する今後の技術開発の課題と方向性, 資源エネルギー庁, 平成27年6月
Pureosity’s Target
The membrane separation process for CO2 has attracted a lot attention, but has yet to achieve wide spread practical use.
The reason for this is that, if you consider the fact that CO2 separation requires a huge amount of processing and for a high yield separation system as described above the performance of the membrane material deteriorates over times.
Up to now, the gas separation membranes that utilize a variety of separation techniques have been developed, but they require both low cost and stability, currently membranes do not have both of these qualities and the ability to modularize the membranes has been hindered. Polyimide and cellulose films have been studied for a long time and are used in the most inexpensive gas separation that are being promoted at the moment. These polymer films tend to have low gas permeability and a large membrane surface area is required for gas processing.
In Pureosity we are developing microporous polymers. We are researching a polymer with a gas molecular sieve structure which internally has the shape of an hourglass. Using this polymer we have achieved a gas permeability as many as 100 to 1000 times that of conventional membrane materials.
However, the gas permeability and gas separation accuracy is to be in a trade-off relationship, and we are continuing research to improve this balance.
Pureosity, without sacrificing the high gas permeation rate of a microporous polymer, has achieved to improve its gas separation accuracy.
One of the results is the cross-linking of the micro-porous polymer by special heat treatment (Nature Communications, 2014).
This cross-linked micro-porous polymer (TOX-PIM-1) compared to conventional gas separation membrane, about 100 times the gas permeability, it shows the excellent performance that is also about twice the gas separation accuracy.
In addition, thermodynamic, and also has the chemical stability, contributing to the reduction of membrane replacement costs due to an increase in the life of the membrane material is expected.
Finally, we aim to make gas separation membrane module with our materials for industry.