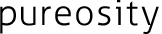What is fouling?
Fouling is the adhesion of contaminants to a surface, typically while under water. Fouling is almost always detrimental and can reduce the efficiency of a surface.
Fouling occurs under a variety of circumstances. For example, the growth of barnacles on the bottom of a ship, the buildup of contaminants on a filter, the buildup of cholesterol in arteries, are all examples of fouling.
Classification of fouling:
Classification “by foulant”
Foulants are basically classified into 3 type, colloids/particles, crystals and biomaterials. The foulant has size variation form colloid with diameter on the order of nanometer to barnacle of order of mm.
Classification by adhesion type
Foulants can adhere onto a surface mechanically by forming a cake or gel, they can also be anchored to the surface through chemical bonds. The cake/gel can be removed by physical washing. Contaminants that are chemically bonded to the surface typically need additional chemical agents, though these risk damaging the surface.
Demand for anti-fouling and its economic effects
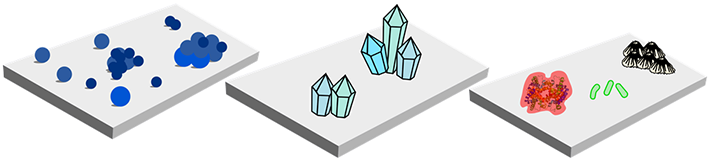
- Fouling can cause the efficiency of membranes and heat exchangers decrease over time. To recover the performance of these devices periodic washing is necessary. Washing has a high cost and can decrease the lifetime of the membrane. For example, it is reported that cost of washing and exchanging membranes can reach 25-30 % of the total running costs of separation.
- It is estimated that fouling materials such as barnacles on the hulls of ships can result in a 9% increase of total transportation cost. Barnacles attach to hulls with a cement type material, which is hard to eliminate.
- Since the economic impact related to fouling is huge, even small percent improvement of anti-fouling methods can be worth it.
Classic Methods of Controlling Fouling
Hydrophilization of Surface
Hydrophilization (increasing the attachment of water to a surface) can prevent adhesion of many foulants, which are typically hydrophobic/oleaginous (don’t like water, like oily or organic surfaces instead).
Paint with Special Chemical Properties
Special paints can reduce fouling. Tributyltin (TBT) has been used in biocidal antifouling paint on the hulls of ships. However, TBT is highly toxic and is prohibited globally now. Environmentally friendly methods are necessary now for novel anti-fouling technology.
Our idea
- Fouling consists of 2 steps: (1) transportation of foulant to the surface; (2) interaction between the foulant and surface. Controlling the flow of water the foulants around the surface has great potential to improve the anti-fouling properties of a surface.
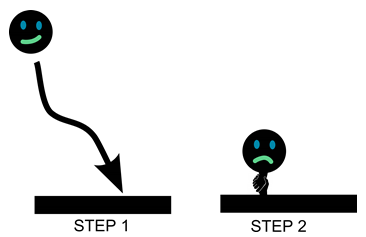
- On flat surfaces, fluid velocity is zero. This means that foulants can easily settle and adhere to the surface.
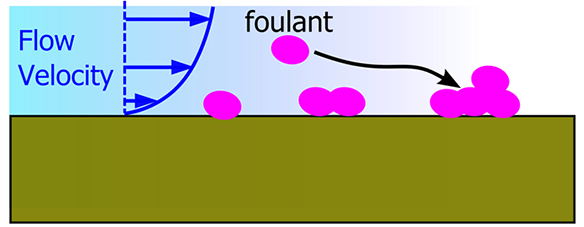
- On some patterned surfaces, water molecules do not stick and a phenomenon known as slip occurs. On nano-patterned surfaces like porous materials there are regions where foulants cannot penetrate. Thus they are kept away by controlling the flow around the patterned surface.
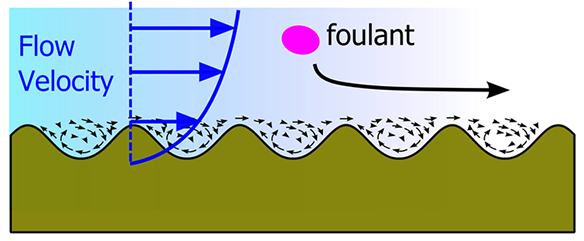
- The design (form and size) must be based on the kind of foulant and the usage conditions. If the surface pattern is larger than foulant, the chance of foulants sticking is higher than on flat surfaces. Our research is focused on the creation of new surfaces and measuring the flow over the surface. We use a special microscope and very sensitive weighing scales to measure the adhesion on our surfaces. Our goal is to understand the relation between different types of water flow and fouling, which is then the guide for designing new materials.