Moisture-Resistant Graphene Oxide Nanolaminates for
Hydrogen Separation Enabled by Nanodiamonds Incorporation
The acceleration of climate change forces us to reduce greenhouse gas emissions urgently. And, as it leaves only water behind when burned, hydrogen (H2) offers a direct way to do so. Several industrial processes produce hydrogen, such as water electrolysis and fossil fuel reforming. But, the produced H2 is not pure and requires further purification. In water electrolysis, hydrogen should be separated from oxygen. On the other hand, fossil fuel conversion includes several major contaminants, such as carbon dioxide. And, in either case, humidity is inescapable.
Unlike conventional separation operations, membrane separation systems require no additional chemicals or involve phase change. They are also easy to scale up and energy-efficient and thus promising for various gas and liquid separation applications. Nevertheless, membrane materials need to be optimized according to specific processes to achieve high separation efficiency and ensure operational stability. For example, in addition to high H2 sieving ability, which is challenging due to the low affinity of H2 to conventional membrane materials, humidity-resistant is a critical factor in designing membrane materials for H2 separation.
 Our membrane research group at Kyoto University actively explores new materials chemistries for developing membranes to address various separation needs. Importantly, we always try to test the capabilities of membrane materials under more realistic conditions. We think it is notably more valuable to consider the impact of envisioned process conditions while exploring the limits of new membrane materials.
Our membrane research group at Kyoto University actively explores new materials chemistries for developing membranes to address various separation needs. Importantly, we always try to test the capabilities of membrane materials under more realistic conditions. We think it is notably more valuable to consider the impact of envisioned process conditions while exploring the limits of new membrane materials.
After the discovery of graphene, there has been a great interest in the development of two-dimensional (2D) materials for gas and liquid separations. With their atomic thicknesses and micrometer-level lateral sizes, graphene-based materials are an emerging platform for designing advanced nanomembranes. Unlike ordinary membranes, the membranes formed from 2D materials can theoretically be as thin as a single atom to enable minimum transport resistance and maximum permeation flux. Sub-nanometer pores derived from interlayer spacing or the controllable assembly of 2D materials allow for highly selective transport of H2 through the membranes.
Among several recently developed advanced graphene-based membranes, graphene oxide (GO) membranes are the most promising. However, the development of nanometric GO membranes is always challenged by the moisture instabilities of GO sheets. The interlayer spacing of the GO membrane would be expanded when the membrane is exposed to water vapor. This swelling effect is associated with the hydration of oxygen-containing groups on the GO nanosheets and results in the disintegration of the membrane’s structure.
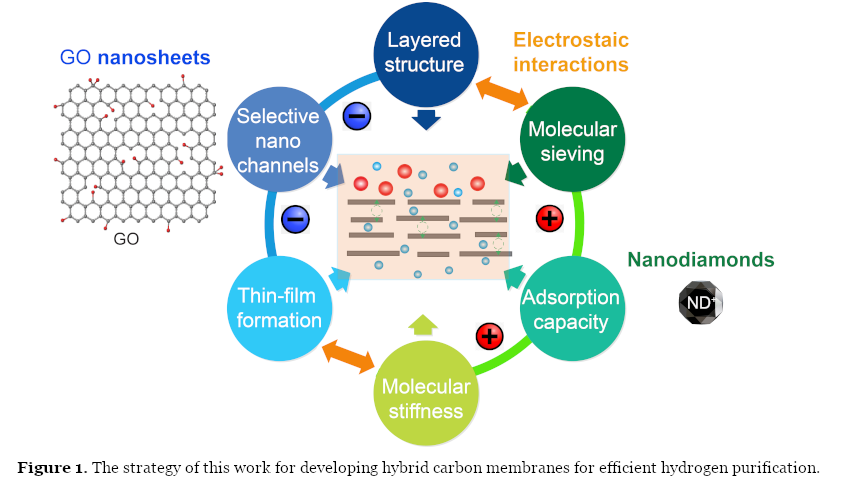
Our recent paper published in Nature Energy targets GO membranes’ stabilization against humidity while maintaining high H2 purification performance. To do so, positively charged nanodiamonds (ND+) were intercalated into negatively charged GO nanosheets. The electrostatic interactions between ND+ and negatively charged GO surfaces, leading to robust ultrastructures even under harsh humid conditions (Figure 1).
In addition to water stability, the inclusion of ND+s increases the overall pore volume and reduces the layer number of well-stacked GO domains, making the membrane structure more accessible for molecular transport (higher permeance). Thus, the superiority of GO/ND+ composite membranes over neat GO membranes is through the manipulation of the GO pore architecture (dimensions, tortuosity, and stiffness) using ND+s.
The highly stable laminated composites developed in this work might also be attractive for other energy and environmental applications such as micro supercapacitors, fuel cells, and sensors.
For more details, please see the full article on the Nature Energy website: www.nature.com/articles/s41560-021-00946-y